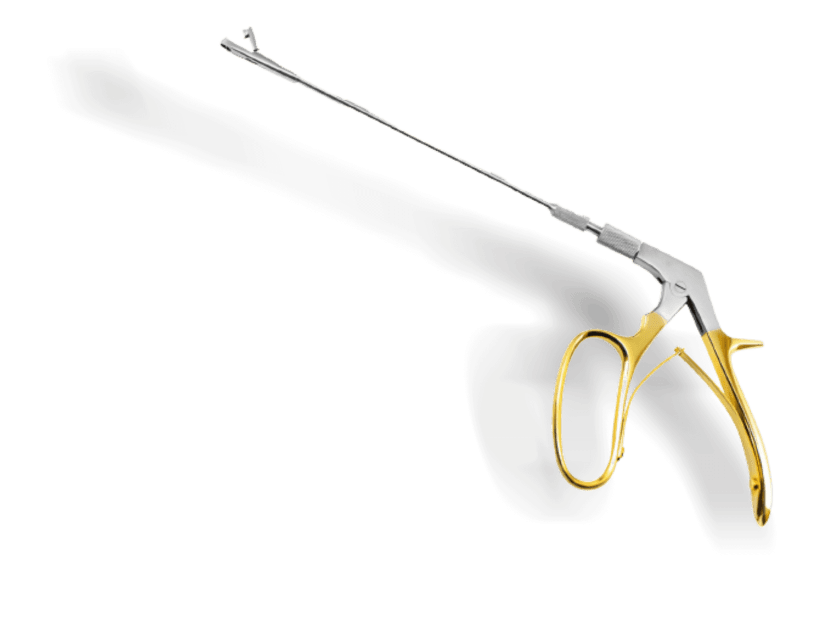
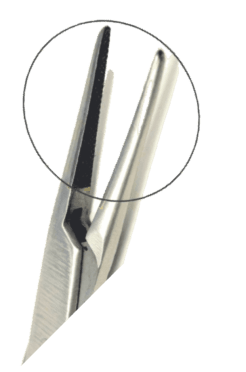
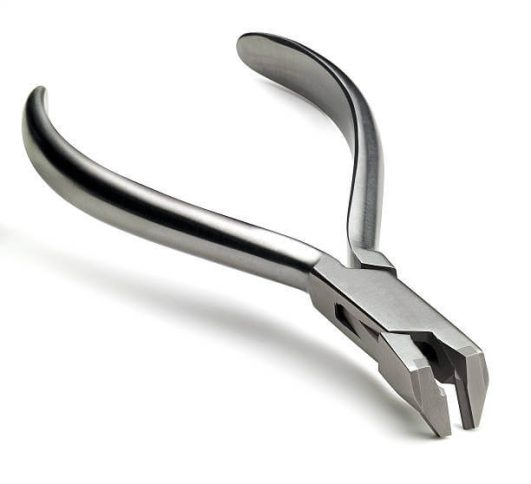
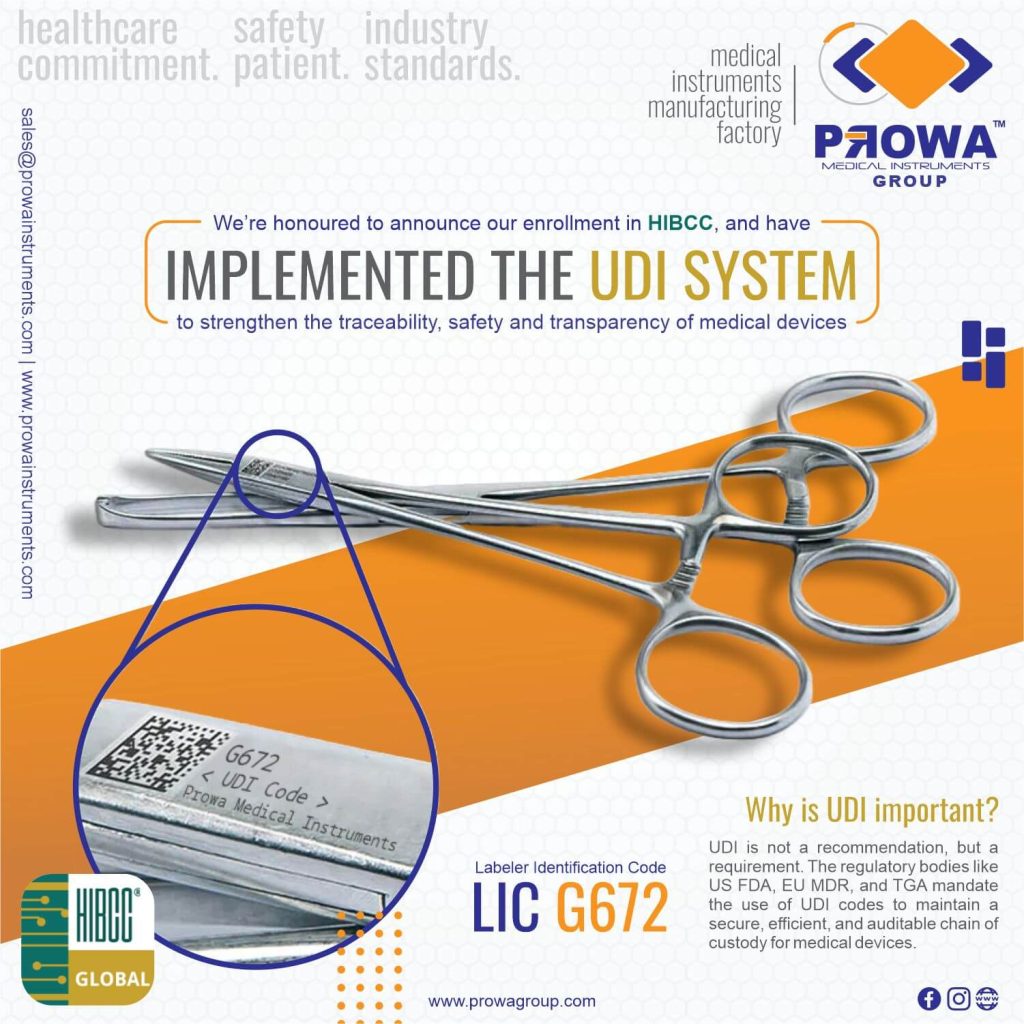
Prowa Medical Instruments Group
Embark on a journey of unparalleled sophistication as we haved extended our footprints to over 03 continents and striving to capture the rest of world, delivering opulence and precision to the most discerning clientele worldwide. We invite individuals and companies to come on board with us in journey to serve the humanity.
Ready to commence a worldwide exploration of our network?